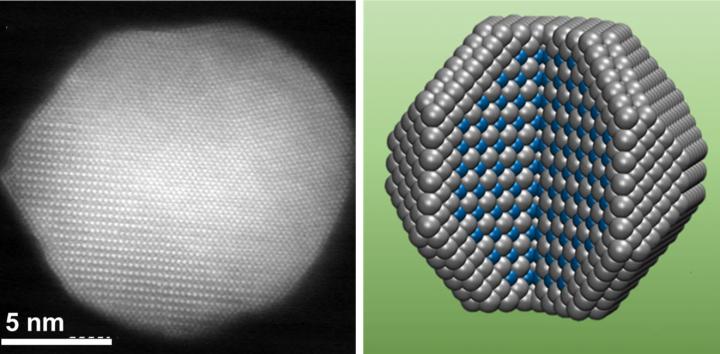
Researchers at Brown University have created a new alloy catalyst that reduces fuel cell costs and beats durability and reactivity standards for 2020.
Less Costly Catalyst
Creating a Less Costly CatalystThe US transportation industry accounted for 28% of greenhouse gas emissions in 2016, according to the Environmental Protection Agency, a problem which could be mitigated through use of eco-friendly hydrogen fuel cells. However, platinum used as the catalyst for these cells is very expensive, and other alloys that initially show performance may oxidize and leach away after normal use.Researchers at Brown University have been studying this problem and have developed a new alloy catalyst that will reduce costs and in tests has outperformed U.S. Department of Energy (DOE) targets for the year 2020 for both reactivity and durability, according to research published in the journal Joule. “This work solves the previous stability issue of the platinum-based alloys under fuel cell conditions,” said Junrui Li, a graduate student in chemistry at Brown and the study’s lead author, in an interview with Innov8 Updates. “It demonstrates how to increase the stability of the alloy catalysts by controlling the structure/morphology of nanocatalysts and how to transfer good test results from labs into a real-life fuel cell device with excellent performance,” he said. In the lab, Li and his colleagues developed alloy nanoparticles with a specialized structure. The particles have a pure platinum outer shell surrounding a core made from alternating layers of platinum and cobalt atoms. That layered core structure is key to the catalyst’s reactivity and durability, said Shouheng Sun, professor of chemistry at Brown and senior author of the research, in a Brown University news release.  “The layered arrangement of atoms in the core helps to smooth and tighten platinum lattice in the outer shell,” Sun continued. “That increases the reactivity of the platinum and at the same time protects the cobalt atoms from being eaten away during a reaction. That’s why these particles perform so much better than alloy particles with random arrangements of metal atoms.” After the experimental lab work, the researchers sent the catalyst to the Los Alamos National Lab for testing as an actual fuel cell to find out how well the catalyst would hold up in a real-world environment. The testing showed that the catalyst beats targets set by the DOE for both initial activity and longer-term durability. The researchers have applied for a provisional patent on the catalyst, and they hope to continue to develop and refine it. The concept may also be transferable to other fuel systems, according to Li. “Our catalyst should be equally effective at the cathode of methanol fuel cells. Hydrogen and methanol oxidation occur at the anode, and they have faster kinetics compared to the cathode reaction (oxygen reduction reaction in this work),” Li said to Innov8 Updates. “This catalyst can also be used in other types of proton exchange membrane fuel cells that use formic acid, ethanol, and other fuels.” According to Li, the researchers will continue to develop catalysts with more improved activity to further reduce the usage of platinum in the catalyst so that they can further decrease the cost, making them more commercially attractive. He said that the research inside their labs may have scale-up potential for outside manufacturing sites. Works Consulted Energy.Gov. Office of Energy Efficiency & Renewable Energy. Fuel Cells. Accessed October 18, 2018. Brown University. News from Brown. New, durable catalyst for key fuel cell reaction may prove useful in eco-friendly vehicles. Accessed October 19, 2018. Li J, Sharma S, Liu X, et al. Hard-Magnet L10-CoPt Nanoparticles Advance Fuel Cell Catalysis. Available online October 16, 2018. Accessed October 19, 2018. |
Even after 30,000 cycles, our catalyst still exceeded the DOE target for initial activity. That kind of performance in a PROFESSOR real-world fuel cell environment is really promising.” |
Less Costly Catalyst May Help Boost Adoption of Eco-Friendly Vehicles
10/19/2018
Researchers at Brown University have created a new alloy catalyst that reduces fuel cell costs and beats durability and reactivity standards for 2020.